Pouch Bag Studies
The Use of Pouch Bags to Learn about Reactions between Electrodes and Electrolyte
The charged electrodes in a Li-ion cell react with the electrolyte. Reaction products created at one electrode can move to the other and be changed by reactions there. Therefore, the presence of both electrodes in a Li-ion cell makes it very difficult to understand the reactions that occur. Placing only a single electrode in âpouch bagâ along with electrolyte (Figure 1) allows individual reactions to be studied without confusion. Pouch bags are normally stored at elevated temperature for several weeks before the electrolyte, evolved gases and electrodes themselves are analyzed. For details, please consult reference 1.
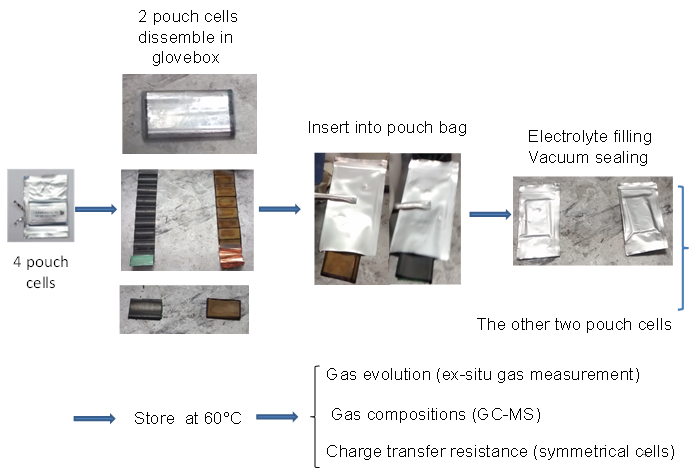
Figure 1: Shows how electrodes are loaded into pouch bags and describes the experiments done on the electrolyte, the gases evolved and the electrodes after storage
Reference:
- D. J. Xiong, R. Petibon, M. Nie, L. Ma, J. Xia, and J. R. Dahn, J. Electrochem. Soc., 163, A546âA551 (2016).
