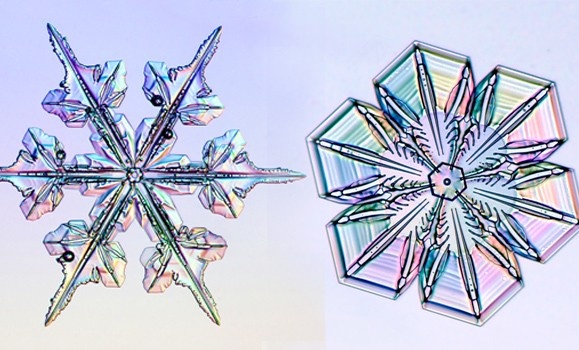
Big ones, little ones, fat ones, sharp ones, round ones: snowflakes come in all sizes and shapes and no two are ever alike. Ever wonder why that is? Kenneth Libbrecht has the answer.
On January 15, Dr. Libbrecht, professor of physics of CalTech, came to H¬ĢĽ≠ to deliver a fascinating lecture titled "The Secret Life of a Snowflake: A Close Look at the Beauty and Mystery in a Crystal of Ice."
A world-renowned expert in crystallization, Dr. Libbrecht is sometimes dubbed "Dr. Snow," and served as a consultant to Disney animators on the hit film Frozen, offering his insight into how snowflakes form.
Up close and personal
Dr. Libbrecht‚Äôs lecture, held in the Goldberg Computer Science Building, was a mix of science, humour and art. Introduced by Mary Anne White of Dal's Department of Chemistry, Dr. Libbrecht began his lecture with pictures of snow, but emphasized his interest in the individual flakes, rather than what he called, ‚Äúbulk snow.‚ÄĚ
‚ÄúI like to photograph snowflakes," he said. "It‚Äôs one of my little hobbies, and there are a couple of ways to do this, but I use a microscope and a camera and I put all this in a suitcase because I happen to live in Southern California ‚ÄĒ a funny place to live when you like to photograph snowflakes.‚ÄĚ
He says that the best temperature for particularly photogenic snowflakes is about -15 Celsius. He shared his photos while explaining the process of sublimation, and showing how the snowflakes melt and evaporate on the microscope slide. Through his photos, he explained how snowflakes form and some of the science behind them.
Clouds are full of tiny water droplets ‚ÄĒ so small they don‚Äôt fall from the sky. When it gets below freezing, those droplets freeze, collecting the water vapour around them and growing in shape and size. As they get bigger, they become heavier and then fall to the earth.
Unique creations
So what makes each snowflake different?
As Dr. Libbrecht explains it, ‚ÄúNo two snowflakes take the same path down from the clouds, and their water molecules form according to that path.‚ÄĚ
As for how he conducts his snowflake research in such a warm location, Dr. Libbrecht is able to make artificial snowflakes in his lab in California. In a tank, his team uses water vapour to create artificial clouds and, consequently, snowflakes.
“It allows us to study the snowflake as it is taking shape," he said.
You can see more of Dr. Libbrecht’s snowflake photos .
His lecture was co-sponsored by the Institute for Research in Materials, the Department of Physics and Atmospheric Science, and the Department of Chemistry.